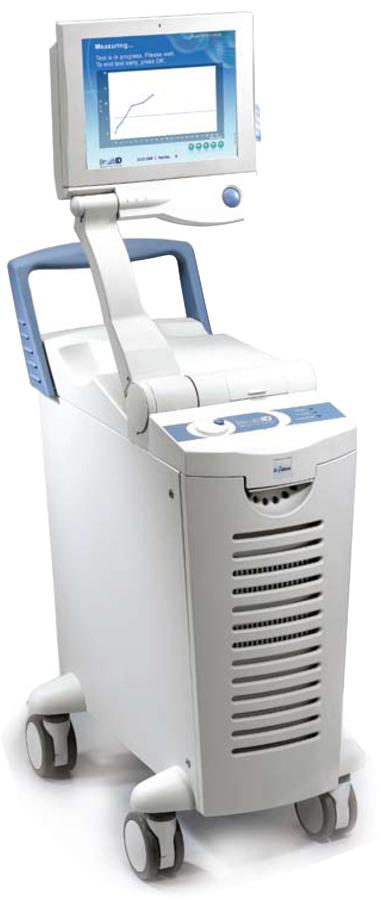
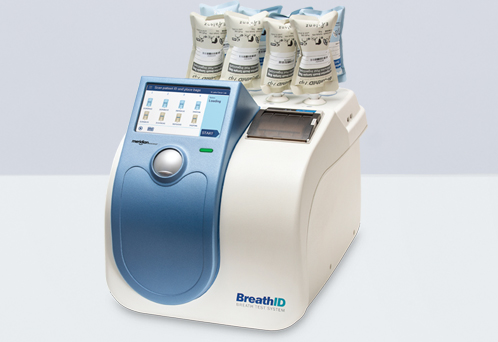
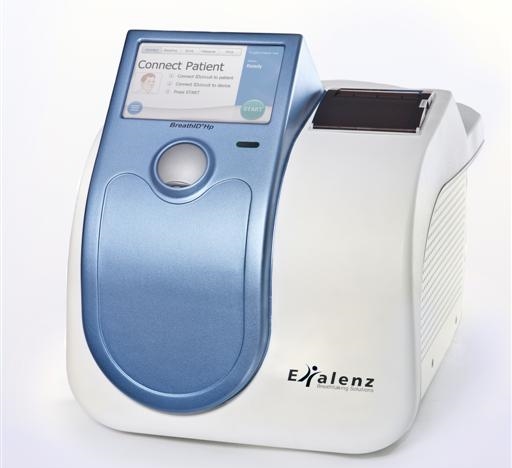
Breathid Breath Test System
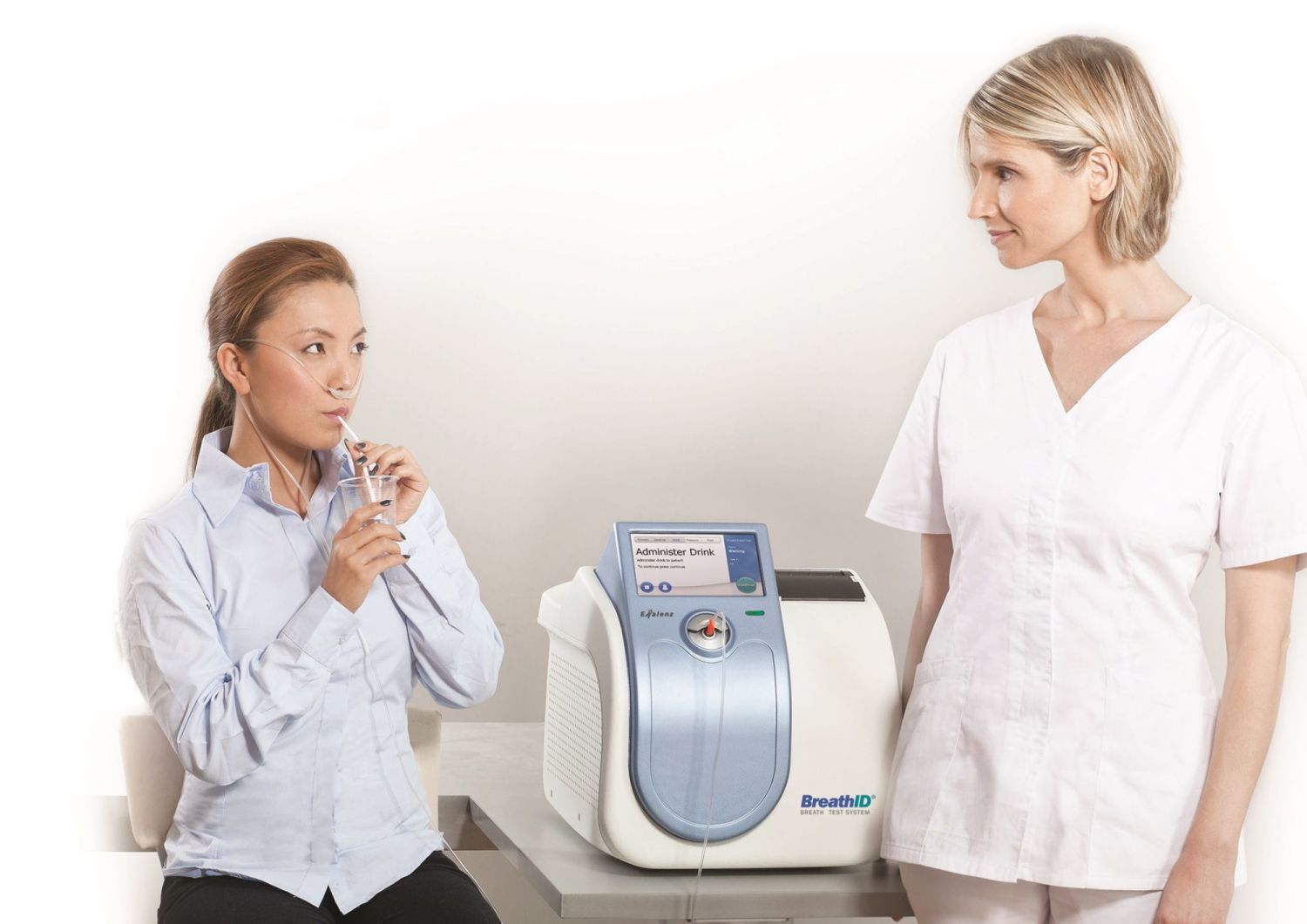
A 75 mg 13c urea tablet and 4 3 g citrica powder are dissolved in water.
Breathid breath test system. The exalenz breathid non invasive breath test is a diagnostic test that analyzes a breath sample before and after ingestion of 13c enriched urea. Blood chemistry leadcare proficiency and controls human conditions. The breathid system is a revolutionary platform capable of assessing a range of liver and gastrointestinal disorders via molecular analysis of the patient s exhaled breath breathid uses a clinically proven sophisticated laser like light source to pinpoint real time changes in 13c 12c isotope ratios at an accuracy level of single parts per. Other applications are limited in the us by federal law to investigational use only.
The exalenz breathid breath test is performed as follows. It is used to identify those patients with h. The urea breath test is used to detect helicobacter pylori h. Urea breath platforms breathid hp lab breathid hp breathid smart.
In the us the breathid hp system and the breathid hp lab system are intended for use in the qualitative detection of h. The exalenz breathid non invasive breath test is a diagnostic test that analyzes a breath sample before and after ingestion of 13c enriched urea. Yet many patients remain unknowingly infected and often untested or masked over with ppi prescriptions. Immunoassay curian immunocard stat immunocard premier tru merifluor immunodiffusion latex agglutination quikpac ii genbody.
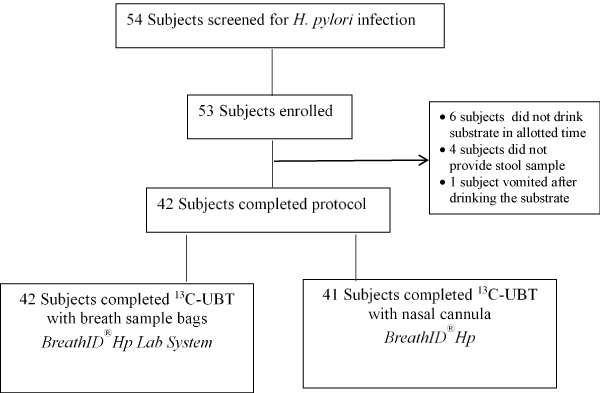
The stool sample will be provided within a week of the breath tests and will be analyzed by a central laboratory. The need for testing and eradicating is real labeled a carcinogen by the world health organization h. A 75 mg 13c urea tablet and 4 3 g citrica powder are dissolved in water. The exalenz breathid breath test is performed as follows.
Pylori if left untreated is a major cause of ulcers and strongly linked to stomach cancer. They have fda 510 k clearance and an approved nda for the 13 c urea tablet and citrica powder.